Introduction
Age-related macular degeneration (ARMD) is the most common maculopathy causing visual impairment in those aged over 75 years.1–3 It begins with drusen (lipofuscin deposits) and may progress to central vision loss through dry…

Age-related macular degeneration (ARMD) is the most common maculopathy causing visual impairment in those aged over 75 years.1–3 It begins with drusen (lipofuscin deposits) and may progress to central vision loss through dry…

Diabetic retinopathy (DR), an ocular microvascular complication of diabetes, is a leading cause of visual impairment and blindness among working-age individuals.1 An estimated 103 million individuals are currently living with DR worldwide, and this number is projected to rise to 160 million by 2045, driven by an aging population and improved survival rates among individuals with diabetes.2 Between 1990 and 2015, DR-related blindness increased from 200,000 to 400,000 cases and visual impairment from 1.4 million to 2.6 million, reflecting a steady increase in global prevalence that poses a critical public health challenge.3 Treatment decisions for DR are often complex, as patients face a variety of options, including retinal laser photocoagulation, anti-vascular endothelial growth factor (anti-VEGF) drugs, corticosteroids, and pars plana vitrectomy.4 These treatment options differ in their efficacy, risks, economic burden, and impact on daily life.5 Improper decision-making may lead to severe consequences, including significant vision loss, irreversible blindness, and other serious secondary complications.5,6 Evaluating these complex, high-stakes trade-offs presents significant challenges to decision-making for patients with DR.
The World Health Organization (WHO) advocates for patient involvement in clinical decision-making to uphold patients’ rights to participate in their treatment plans and maximize treatment benefits.7 Previous studies have demonstrated that patient participation in treatment decision-making can not only improve treatment adherence and reduce medical visits but also enhance the doctor-patient relationship and improve health outcomes.8,9 Therefore, involving DR patients and incorporating their preferences is essential. However, little is known about patient involvement in DR treatment decisions, especially in the context of the Chinese healthcare system. Compared to many Western countries, Chinese medical culture has traditionally been characterized by a physician-centered model, in which physicians are regarded as authorities and patients often adopt a passive role, with compliance considered optimal.10 Thus, a systematic exploration of the factors influencing patient involvement in DR decision-making in China is warranted to enhance clinical decision quality.
Previous studies suggest that factors such as age, gender, economic status, educational level, health literacy, and social support may influence participation in decision-making.11,12 However, several factors remain controversial. Two studies found that older patients tended to play a passive role in treatment decision-making,13,14 whereas one study reported no significant age-related effects.15 One study found that female patients, compared to men, reported a greater preference for a collaborative role and a lesser preference for a passive role in decision-making,16 while another study demonstrated the opposite conclusion.17 A study indicated that high social support was associated with increased patient participation in surgical decision-making.18 In contrast, a qualitative study revealed that family support, a component of social support, sometimes hindered patient involvement and, in some cases, led to family members making decisions on the patient’s behalf.19 These contradictory findings underscore the necessity for a more systematic and theoretically grounded approach to understanding patient involvement.
To fully capture the influencing factors, a comprehensive theoretical framework is essential. The Capability, Opportunity, Motivation – Behavior (COM-B) model offers such a framework.20 Widely recognized for its comprehensive and systematic approach, the COM-B model has been extensively applied to understand a range of patient health behaviors.21–23 The model posits that an individual’s behavior is a result of their capability, opportunity, and motivation, with capability and opportunity also affecting behavior both directly and indirectly through their impact on motivation.20
Through literature review and group discussions, we identified a set of potential determinants which were then mapped onto the COM-B framework. Capability is defined as an individual’s psychological and physical ability to perform a behavior. Health literacy reflects the patient’s ability to access, comprehend, and utilize health information.24 This ability allows patients to understand their treatment options, evaluate risks and benefits, and communicate their preferences meaningfully. In this study, capability was conceptualized as health literacy. Opportunity encompasses physical and social factors that facilitate or prompt a behavior. After seeking medical attention, physicians often serve as the primary source of medical information for patients in China.25 Research has shown that physician support is a crucial facilitator for patient involvement in treatment decisions.26 Furthermore, social support from interpersonal networks, including family members and friends, can enhance patients’ psychological resilience and mitigate decision-making pressure.27 For this study, we conceptualized ophthalmologist facilitation of patient involvement and social support as opportunity. Motivation refers to the internal brain processes that energize and direct behavior, including both reflective and automatic mechanisms. Patients with higher decision self-efficacy are more likely to seek information, express their preferences, and play a more engaged role in the treatment process.28 Additionally, the need for decision-making involvement reflects patients’ intrinsic desire or preference for participation. In this study, we measured motivation through decision self-efficacy and the need for decision-making involvement. Figure 1 illustrates the conceptual framework that guided our study.
|
Figure 1 The conceptual framework guiding the study.
|
Given that constructs such as health literacy, social support, and need for decision-making involvement are complex and multifaceted, we conducted our analysis on their respective sub-dimensions. For example, we analyzed the functional, communicative, and critical sub-dimensions of health literacy. This approach enabled us to more comprehensively understand how different layers of capability, opportunity, and motivation collectively influenced patient decision-making behavior. The aims of this study were to investigate the current status of actual involvement roles in treatment decision-making among patients with DR and to analyze the influencing factors. The research results will provide a valuable reference for developing measures to promote patient involvement in decision-making.
This cross-sectional study was conducted at the ophthalmology center of a large public hospital in Shanghai, China, from August 2024 to January 2025. The institution serves as a regional referral center for a broad geographic area encompassing Shanghai municipality and the surrounding provinces of Jiangsu, Zhejiang, and Anhui. The study participants were recruited using a convenience sampling method. Participants meeting the following criteria were included: (1) age 18 years and above; (2) diagnosed with DR stages III to VI; (3) voluntary participation in the study and signed informed consent. Patients with mental illness, intellectual disability, or verbal communication disorders, as well as severe cardiac, hepatic, or renal dysfunction, respiratory failure, or critical illness, were excluded from the study. According to the Kendall sample size estimation method, which is calculated based on the principle that the sample size should be at least 5 to 10 times the number of variables.29 Through a literature review, this study included a total of 24 predictive influencing variables, comprising 13 sociodemographic and disease characteristics and 11 variables from five scales. Considering a 10% attrition rate, the calculated total sample size of this study ranged from 134 to 267 cases. Ultimately, the study obtained 336 valid samples. This study was reported using the STROBE guidelines.
This study was performed in line with the principles of the Declaration of Helsinki. Ethical approval was granted by the Ethics Review Committee of Shanghai General Hospital (Approval No. 2024–098). All participants provided written informed consent and retained the right to withdraw at any time. All data were collected and analyzed anonymously to guarantee confidentiality.
The questionnaire was designed based on a literature review and consultation with ophthalmology experts. It includes the following information: gender, age, marital status, educational level, monthly per capita household income, method of healthcare payment, duration of disease diagnosis, DR stage, and comorbidities.
The CPS is used to assess actual roles in treatment decision-making among patients with DR. The scale was originally developed by Degner and later adapted and revised by Nolan.30 Chinese scholar Xu Xiaolin and colleagues translated and revised the scale, and the Cronbach’s α coefficient of the Chinese version is 0.899.31 The CPS is a unidimensional scale consisting of five options to characterize the types of patient involvement in treatment decision-making. Options 1 and 2 represent the active type, option 3 represents the collaborative type, and options 4 and 5 represent the passive type.
The AAHLS is used to assess patients’ health literacy levels. The scale was developed by Chinn in 2013,24 and translated and revised by Wu in 2016.32 It consists of 11 items across three dimensions: functional health literacy, communicative health literacy, and critical health literacy. Each item is scored on a 3-point Likert scale (1 = rarely, 2 = sometimes, 3 = often). The total score ranges from 11 to 33, with higher values indicating greater health literacy. In this study, the Cronbach’s α coefficient of the scale was 0.834.
The SSRS, developed by Xiao, is used to assess patients’ social support levels.33 It includes 10 items across three dimensions: objective support, subjective support, and utilization of social support. The total score ranges from 8 to 44, with higher scores indicating higher social support. In this study, the Cronbach’s α coefficient of the scale was 0.800.
The FPIS is used to measure the extent to which patients perceive that their healthcare professionals involve them in their healthcare, developed by Martin.34 The Chinese version was translated and revised by Wu in 2015.32 It is a unidimensional scale with nine items. Each item is scored on a 6-point Likert scale ranging from 1 (never) to 6 (always). The scale yields a total score ranging from 9 to 54, with higher values reflecting greater healthcare providers’ facilitation of patient involvement in treatment decisions. In this study, the Cronbach’s α coefficient of the scale was 0.830.
The DSES is used to assess patients’ confidence in making treatment decisions for themselves and was developed by O’Conner.35 It is a unidimensional scale comprising eleven items, each rated on a 5-point Likert scale from 0 (not at all confident) to 4 (very confident). The total score is calculated by averaging the sum of 11 items and then multiplying by 25 to convert it to a 0–100 scale. Higher scores reflect greater patient self-efficacy in treatment decision-making. In this study, the Cronbach’s α coefficient of the scale was 0.864.
The PEPMDS, developed by Xu, is used to assess patients’ need for participation in treatment decisions.36 This scale consists of 12 items in three dimensions: need for information, need for deliberation, and need for decisional control. Items are rated on a 5-point Likert scale (1 = strongly disagree to 5 = strongly agree). The total score ranges from 12 to 60, with higher scores indicating greater patient need for involvement in treatment decisions. In this study, the Cronbach’s α coefficient of the scale was 0.846.
Before conducting the questionnaire survey, all research team members received standardized training to ensure the consistency and uniformity of terminology and procedures in the survey. During the distribution of the questionnaires, researchers provided participants who met the inclusion criteria with a detailed explanation of the study’s purpose, content, and procedures, and obtained their written informed consent. Participants’ medical information was collected via the hospital’s electronic medical record system. Questionnaires were distributed and collected on-site, with immediate checks to ensure the completeness and accuracy of data collection. For participants who were unable to complete the questionnaire independently due to visual impairment, the researchers administered the questionnaire via dictation based on their verbal responses.
Data were entered independently by two investigators using EpiData 3.1 and exported to SPSS 26.0 for analysis after verification. Descriptive analyses were performed for all included variables. Frequencies and percentages were used for categorical variables, means and standard deviations (M±SD) were used for normally distributed continuous variables, and medians and interquartile ranges (IQR) were used for non-normally distributed continuous variables. The χ2 test was used to analyze the differences in the types of patient involvement in treatment decision-making between categorical variables. One-way ANOVA or the non-parametric Kruskal–Wallis H-test was used to analyze continuous variables. Variables with a P<0.05 in univariate analysis were subsequently included in the unordered multinomial logistic regression analysis to determine independent factors associated with the types of patient involvement in treatment decision-making. The Variance Inflation Factor (VIF) was used to analyze whether the variables in the model have multicollinearity. A two-sided test was used, and a P-value <0.05 was considered statistically significant.
A total of 362 questionnaires were distributed in this study. Following the exclusion of 26 unqualified questionnaires, 336 valid questionnaires were collected, yielding an effective response rate of 92.8%. The mean age of the participants was 53.74±13.01 years. Among the participants, 52.1% were male, and 40.2% had attained a junior high school education or below. The largest proportion of patients was diagnosed with DR Stage IV, accounting for 47.3%. Patients who received retinal photocoagulation accounted for the largest treatment group, at 30.0%. Furthermore, 56.5% of patients presented with ocular comorbidities, and 62.5% had systemic comorbidities. The sociodemographic and clinical characteristics of the patients are shown in Table 1.
 |
Table 1 Comparison of Sociodemographic and Disease Characteristics Among Patients with Diabetic Retinopathy in Different Decision-Making Involvement Roles (N = 336)
|
Regarding actual patient involvement in treatment decision-making roles, 21.1% of patients reported being active, 30.7% reported being collaborative, and the largest proportion, 48.2%, reported being passive.
The mean scores for the 336 patients with DR were AAHLS (24.84±3.53), SSRS (43.59±5.51), FPIS (40.39±4.78), DSES (72.03±11.44), and PEPMDS (47.89±5.39). Univariate analysis revealed significant differences in actual involvement in decision-making roles among patients with DR across several factors, including: age, educational level, monthly per capita household income, health literacy, social support, ophthalmologist facilitation of patient involvement, decision self-efficacy, and need for decision-making involvement (P<0.05). See Table 1 and Table 2.
 |
Table 2 Comparison of COM-B Factors Among Patients with Diabetic Retinopathy in Different Decision-Making Involvement Roles (N = 336)
|
An unordered multinomial logistic regression analysis was conducted to identify the independent factors influencing patient involvement in treatment decision-making for diabetic retinopathy. Prior to conducting the multinomial logistic regression, multicollinearity was assessed among all independent variables. The aggregate variables social support and need for decision-making involvement demonstrated perfect collinearity with their respective sub-dimensional variables, as indicated by a tolerance value of 0.000. The variable health literacy also exhibited significant collinearity, with a VIF of 11.381. Since the simultaneous inclusion of these aggregate variables and their subdimensions would have rendered the model unstable and statistically un-fittable, they were excluded in accordance with established statistical principles. Consequently, only the sub-dimensional variables were retained in the final model. This approach ensured the robustness of the model while enabling evaluation of the impact of specific components within each theoretical construct on decision-making behavior. After refitting, all remaining variables had VIF values below 4, indicating no substantial multicollinearity. The following variables were retained and subsequently entered into the multinomial logistic regression analysis: age, educational level, monthly per capita household income, functional health literacy, critical health literacy, objective support, subjective support, utilization of social support, need for information, need for deliberation, need for decisional control, ophthalmologist facilitation of patient involvement, and decision self-efficacy. The assigned values of the included independent variables are shown in Table 3.
 |
Table 3 Assignment of Independent Variables
|
The likelihood ratio test indicated that the final model provided a significantly better fit than a null model (χ² = 272.002, df = 32, P < 0.001). This conclusion was bolstered by the pseudo R² values (Cox & Snell R² = 0.555; Nagelkerke R² = 0.634), which collectively suggest that the model captures a substantial proportion of the outcome’s variability and signals a good overall fit. The results identified age, monthly per capita household income, critical health literacy, ophthalmologist facilitation of patient involvement, objective support, and need for deliberation as significant independent predictors of patient decision-making roles. Compared to passive roles, patients adopting active roles tended to be younger, have higher income, report lower ophthalmologist facilitation, and express higher need for deliberation. Conversely, compared to passive roles, collaborative roles were associated with higher income, greater critical health literacy, stronger ophthalmologist facilitation, and elevated need for deliberation. Furthermore, when collaborative roles were compared to active roles, patients adopting the former were significantly older, reported higher objective support, and experienced greater ophthalmologist facilitation. The detailed results are shown in Table 4.
 |
Table 4 Multinomial Logistic Regression Analysis of Factors Influencing the Actual Involvement in Decision-Making Roles Among Patients with Diabetic Retinopathy (n=336)
|
To our knowledge, this is the first study to examine actual treatment decision-making involvement among Chinese patients with DR and explore its influencing factors using the COM-B framework. Overall, the passive role was the most common pattern observed. Furthermore, our analysis identified several key factors significantly influencing patient involvement: age, monthly per capita household income, critical health literacy, ophthalmologist facilitation of patient involvement, objective support, and need for deliberation.
The results revealed that 48.2% of patients reported passive roles, 30.7% reported collaborative roles, and 21.1% reported active roles. The proportion of passive role was relatively higher in patients with DR than in those with inflammatory bowel disease (32.12%),37 gynecologic cancer (29.9%),38 or atrial fibrillation (40.3%).26 These differences may be attributed to distinct study populations and research settings. Specifically, DR has one of the lowest levels of public awareness compared with other ophthalmic diseases, which is likely due to the high professional barriers inherent in its diagnosis and treatment.39 This low awareness is evidenced by a Chinese study where only 1.2% of diabetic patients could correctly identify symptoms of DR.40 Furthermore, the combination of diverse treatment options, prognostic uncertainty, and the older demographics of the patient cohort may collectively impair patients’ understanding of the disease and therapeutic alternatives, thus predisposing them to passive decision-making.
Previous studies have pointed out that health literacy, encompassing various dimensions, is necessary for patient involvement in decision-making.41,42 Successful patient involvement is predicated on patients possessing practical communication skills, the ability to acquire, comprehend, and communicate relevant information about disease and treatment from healthcare professionals, and critical evaluation skills.41 However, few studies have explored the distinct impacts of these different health literacy dimensions on patients’ actual involvement in decision-making. Our findings indicate that patients with higher critical health literacy are more likely to adopt collaborative decision-making roles. This result is consistent with a study conducted in the Netherlands, which found that critical health literacy was more important for patient participation than its functional and communicative counterparts.43 In contrast, a French study reported a positive correlation for functional and communicative health literacy with patient involvement but no association for critical health literacy.44 This discrepancy may be attributable to the relatively homogeneous scores for functional and communicative health literacy in our cohort, which exhibited limited variability. Our research suggests that the ability to simply acquire information and communicate with healthcare professionals is insufficient for meaningful participation in medical decision-making. Therefore, future interventions should focus on enhancing the critical health literacy of patients with DR, empowering them to analyze, evaluate, and question health information to make informed decisions that align with their personal values and preferences.
Our study revealed that increased ophthalmologist facilitation is significantly associated with patients adopting a collaborative role over either a passive or an active one. This suggests that when ophthalmologists proactively provide information, encourage questions, and respect patient concerns, they effectively bridge the inherent information and power asymmetry. Such empowerment fosters a climate of equitable dialogue, promoting patient engagement to collaborate rather than simply shifting the decision-making burden onto them. This finding aligns with a qualitative meta-summary which highlighted that clear information delivery, active listening, and trust-building are critical facilitators for patient engagement.45 Furthermore, our results showed that greater ophthalmologist facilitation decreased the likelihood of an active role. A potential explanation is that highly facilitative ophthalmologists build a strong foundation of trust, engendering a sense of security and understanding in patients. Consequently, the perceived need for patients to assume a solely autonomous, active role diminishes. This perspective is supported by Kraetschmer’s research, which established that an active role is associated with low trust, a passive role with blind trust, and a collaborative role with high but not excessive trust.46 Conversely, when patients perceive a lack of support or transparency, their trust may diminish, compelling them to adopt an active, patient-dominated role as a compensatory strategy to regain a sense of control.47 Physician support, therefore, bridges the information and power gap, enabling true shared decision-making rather than pushing patients toward extremes.48 This is further corroborated by a cross-sectional study which showed that physicians’ facilitative communication predicted greater patient participation.49 Consequently, future interventions should focus on implementing training programs for ophthalmologists to enhance communication skills, integrate appropriate decision aids into practice, and foster patient empowerment and collaboration.
Visual impairment caused by DR leads to significant consequences, including disrupted family functioning, increased social isolation and dependence, and economic constraints, and inadequate social support is common in patients with DR.50 Previous studies have established that social support promotes greater patient participation in decision-making by providing financial, emotional, and informational resources that reduce psychological stress and decision-making conflicts.51–53 However, few studies have specifically evaluated the distinct impacts of different dimensions of social support on patient involvement in decision-making. Our study found that higher objective support was significantly associated with the adoption of a collaborative role in treatment decision-making. Objective support, in this context, refers to the tangible, visible, and practical assistance that patients receive from their social network, such as family, relatives and friends. Our study suggests that objective support is a more robust predictor of a patient’s decision-making role than are subjective support and the utilization of social support. Objective support provides the concrete resources necessary for shared information exchange, preference clarification, and joint deliberation.54 This association is particularly salient in cultural contexts with strong family involvement and collectivist values, where health decisions are often regarded as a shared family responsibility.55 In such settings, adequate objective support helps mitigate unilateral physician dominance while also alleviating the burden of solitary patient decision-making, thereby promoting a collaborative approach. Therefore, healthcare professionals should systematically assess the objective support levels of patients with diabetic retinopathy. Interventions should simultaneously focus on encouraging patients to strengthen their ties with social networks, such as family, relatives, and friends, and guide family members to become engaged partners in the patient’s treatment journey, thereby bolstering the decision-making support available to them.
Need for decision-making reflects an individual’s intrinsic motivation to control the process and outcome of medical decisions.28 Patients with a strong need for decision-making typically desire in-depth disease information, weighing pros and cons, participating in discussions, and having more personal control over treatment decisions. Charles et al categorized the treatment decision-making process into three components: information exchange, deliberation, and decision-making control.56 The three dimensions of the PEPMDS correspond to the treatment decision-making process.57 The univariate analysis results in our study indicate that all three dimensions are related to the patient’s decision-making role. In the multinomial logistic regression model, only the need for deliberation remained statistically significant. Our research suggests that the need for deliberation is a better predictor of patients taking a collaborative or active role in decision-making than the need for information and decision control. Logically, patients necessarily need access to sufficient medical information if they are to deliberate, and crucially, the process of deliberation often serves as a means for patients to strive for or achieve decision control. Therefore, when the need for deliberation is included in the model, it may have already captured most of the variation explained by the need for information and decision control. This finding suggests that patients, even if well-informed and possessing control, may still choose passive decision-making if they do not have the will to deliberate. Rather than just passively providing information or asking the patient about their willingness to take control of the decision, it is more important to identify whether patients are willing to think deeply, weigh the pros and cons, and discuss with healthcare professionals.
Our research indicated that as patients age, those with diabetic retinopathy were more likely to adopt a passive or collaborative role in decision-making. This finding aligns with Salm’s study,58 while it conflicts with the null results reported by Xie.15 Several contextual factors might explain this discrepancy. In contrast to Xie’s research, which involved undergraduate students and community-dwelling older adults in the United States and focused on general health decision-making scenarios, our work specifically examined patients with sight-threatening diabetic retinopathy. These patients face complex, urgent decisions, such as choosing intravitreal injections or laser surgery that directly affect their vision. For older adults, who may experience age-related cognitive decline or feel overwhelmed by technical medical information, the perceived complexity and high stakes of these decisions likely diminish their capacity to adopt proactive decision-making.59 Furthermore, in the cultural context of our study, younger patients, compared with older patients influenced by traditional paternalistic norms in the doctor-patient relationship, tend to place greater emphasis on asserting personal autonomy and maintaining control over medical decisions.60 Therefore, the effect of age may not be a simple main effect but is likely moderated by the disease context, the nature of the treatment decisions, and the cultural environment. We found no statistically significant association between gender and decision-making roles, a finding that stands in contrast to studies which concluded that females were more participatory or males were more involved.16,17 This divergence suggests that the purported effects of gender may not be a stable, universal phenomenon but are likely contingent upon specific contextual moderators. Overall, there was no consistent evidence to support associations between decision-making roles and gender. Our findings presented that patients with a monthly per capita household income of ≤3000 yuan and 3001–5000 yuan were more inclined to take a passive role in actual decision-making for diabetic retinopathy compared to those with higher incomes. Similar to our results, Wang et al reported that patients with a monthly per capita household income of 5000–7999 yuan exhibited a greater tendency for collaborative decision-making than low-income patients.37 This socioeconomic disparity in decision-making involvement is particularly consequential, given that diabetic retinopathy requires lifelong management involving significant ongoing costs. Treatments such as anti-VEGF therapy, while effective, are relatively expensive. In China, access to these therapies is further constrained by medical insurance policies requiring strict clinical indications and imposing limits on the cumulative reimbursable doses. Consequently, patients with lower household incomes often face substantial financial toxicity, which forces them to weigh treatment costs against potential efficacy and experience heightened psychological distress. This economic pressure frequently leads them to adopt a passive stance, accepting the more economical options recommended by physicians.61 In contrast, patients with higher incomes are less burdened by cost considerations. Their relative financial security, coupled with potentially greater access to comprehensive health information and support services, reduces the perceived burden of decision-making and fosters greater participation in choosing their treatment options. These observed disparities underscore the critical need for reforms in the national medical insurance payment system. Such reforms should aim to alleviate the financial barriers that currently prevent equitable access to preferred treatments and hinder the full participation of socioeconomically disadvantaged patients in treatment decision-making.
The study has two primary strengths. First, its theory-driven approach, underpinned by the COM-B model, allowed for a systematic and integrated analysis of the determinants of patient involvement, offering clear targets for future interventions. Second, its comprehensive assessment of key variables within the specific, high-stakes context of diabetic retinopathy enhanced the clinical relevance and applicability of the findings. This study has several limitations. First, the generalizability of our findings is limited by the sample, which was not only relatively small in size but also drawn from a single tertiary hospital in Shanghai. This specific context may not be representative of broader populations, particularly those in community clinics or rural settings where patient demographics and healthcare resources differ considerably. Second, the cross-sectional design cannot establish causal relationships between variables. Longitudinal studies are necessary to untangle these complex temporal dynamics and verify the directionality of these associations. Third, the reliance on self-reported data may introduce social desirability and recall biases, potentially affecting measurement validity. Future studies could incorporate objective measures, such as audio recordings of clinical encounters, to improve accuracy. Finally, our analytical strategy of using sub-dimensions limited our ability to quantify the overall impact of the integrated COM-B constructs. Given these limitations, the findings should be interpreted as exploratory and require validation in larger, more diverse cohorts. Future large-scale studies will also be better equipped to employ advanced statistical modeling techniques to further elucidate these complex relationships.
This study, the first to apply the COM-B model to decision-making involvement among DR patients in China, revealed a predominance of passive involvement among patients. Our findings suggested several potential determinants consistent with the COM-B framework, including capability factors such as critical health literacy, opportunity factors including ophthalmologist facilitation of patient involvement and objective support, and motivation factors like the need for deliberation, in addition to demographic variables such as age and income. These findings underscore the necessity for multifaceted interventions, including tailored patient education programs, clinician communication training, and accessible decision aids, to promote shared decision-making. However, given the constraints of the cross-sectional design and limited sample size, these conclusions should be considered exploratory. Future research should focus on large-scale longitudinal studies to track changes in patient decision-making roles over time, as well as intervention trials assessing the effectiveness of COM-B-based strategies in promoting shared decision-making.
DR, diabetic retinopathy; COM-B, capability, opportunity, motivation, and behavior; CPS, control preference scale; AAHLS, all aspects of health literacy scale; SSRS, social support rating scale; FPIS, facilitation of patient involvement scale; DSES, decision self-efficacy scale; PEPMDS, patient expectation for participation in medical decision-making scale.
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Shanghai General Hospital (Approval No. 2024-098). Written informed consent was obtained from all participants.
The authors wish to thank all the patients and staff who participated in this study.
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
The research did not get any dedicated financial funding from public, commercial, or not-for-profit funding organizations.
The authors report no conflicts of interest in this work.
1. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi:10.1016/s0140-6736(09)62124-3
2. Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580–1591. doi:10.1016/j.ophtha.2021.04.027
3. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. doi:10.1016/s2214-109x(17)30393-5
4. Raj A, Singla A, Sidana S. Preventive and therapeutic strategies via health care delivery system to minimize sight-threatening diabetic retinopathy: a narrative review. Curr Diab Rep. 2025;25(1):36. doi:10.1007/s11892-025-01591-5
5. Shughoury A, Bhatwadekar A, Jusufbegovic D, Hajrasouliha A, Ciulla TA. The evolving therapeutic landscape of diabetic retinopathy. Expert Opin Biol Ther. 2023;23(10):969–985. doi:10.1080/14712598.2023.2247987
6. Salvetat ML, Pellegrini F, Spadea L, et al. The treatment of diabetic retinal edema with intravitreal steroids: how and when. J Clin Med. 2024;13(5). doi:10.3390/jcm13051327
7. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4(4):Cd001431. doi:10.1002/14651858.CD001431.pub5
8. Becker C, Gross S, Gamp M, et al. Patients’ preference for participation in medical decision-making: secondary analysis of the BEDSIDE-OUTSIDE trial. J Gen Intern Med. 2023;38(5):1180–1189. doi:10.1007/s11606-022-07775-z
9. Ruhnke GW, Tak HJ, Meltzer DO. Association of preferences for participation in decision-making with care satisfaction among hospitalized patients. JAMA Network Open. 2020;3(10):e2018766. doi:10.1001/jamanetworkopen.2020.18766
10. Li B. The power paradox of patient-centred care in Chinese community health: towards a conceptualisation. Soc Sci Med. 2025;371:117883. doi:10.1016/j.socscimed.2025.117883
11. Tang C, Wang A, Yan J. Exploring motivations and resistances for implementing shared decision-making in clinical practice: a systematic review based on a structure-process-outcome model. Health Expect. 2022;25(4):1254–1268. doi:10.1111/hex.13541
12. Truglio-Londrigan M, Slyer JT, Singleton JK, Worral P. A qualitative systematic review of internal and external influences on shared decision-making in all health care settings. JBI Libr Syst Rev. 2012;10(58):4633–4646. doi:10.11124/jbisrir-2012-432
13. Singh JA, Sloan JA, Atherton PJ, et al. Preferred roles in treatment decision making among patients with cancer: a pooled analysis of studies using the Control Preferences Scale. Am J Manag Care. 2010;16(9):688–696.
14. Fischer M, Visser A, Voerman B, Garssen B, van Andel G, Bensing J. Treatment decision making in prostate cancer: patients’ participation in complex decisions. Patient Educ Couns. 2006;63(3):308–313. doi:10.1016/j.pec.2006.07.009
15. Xie B, Wang M, Feldman R, Zhou L. Exploring older and younger adults’ preferences for health information and participation in decision making using the Health Information Wants Questionnaire (HIWQ). Health Expect. 2014;17(6):795–808. doi:10.1111/j.1369-7625.2012.00804.x
16. Lechner S, Herzog W, Boehlen F, et al. Control preferences in treatment decisions among older adults – results of a large population-based study. J Psychosom Res. 2016;86:28–33. doi:10.1016/j.jpsychores.2016.05.004
17. Torrente-Jimenez RS, Feijoo-Cid M, Rivero-Santana AJ, et al. Gender differences in the decision-making process for undergoing total knee replacement. Patient Educ Couns. 2022;105(12):3459–3465. doi:10.1016/j.pec.2022.08.014
18. Tang H, Dong S, Wang S, et al. Perceived participation in decision-making on primary surgery and associated factors among early breast cancer patients: a cross-sectional study. Cancer Nurs. 2023;46(2):111–119. doi:10.1097/ncc.0000000000001071
19. Jiang Y, Guo J, Sun P, et al. Perceptions and experiences of older patients and healthcare professionals regarding shared decision-making in pulmonary rehabilitation: a qualitative study. Clin Rehabil. 2021;35(11):1627–1639. doi:10.1177/02692155211010279
20. Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. doi:10.1186/1748-5908-6-42
21. Shi Y, Xie XY, Lao AD, Shao L, Wang ZA, Zhang JE. Prevalence of physical inactivity and its determinants among older adults living in nursing homes: a cross-sectional study based on COM-B model. J Clin Nurs. 2025;34(1):204–217. doi:10.1111/jocn.17325
22. Zhang M, Guo L, Namassevayam G, et al. Factors associated with health behaviours among stroke survivors: a mixed-methods study using COM-B model. J Clin Nurs. 2024;33(6):2138–2152. doi:10.1111/jocn.17103
23. Shpendi S, Norman P, Gibson-Miller J, Webster R. Cervical screening attendance in young women and people with a cervix: an application of the COM-B model. Br J Health Psychol. 2025;30(3):e70016. doi:10.1111/bjhp.70016
24. Chinn D, McCarthy C. All Aspects of Health Literacy Scale (AAHLS): developing a tool to measure functional, communicative and critical health literacy in primary healthcare settings. Patient Educ Couns. 2013;90(2):247–253. doi:10.1016/j.pec.2012.10.019
25. Lu L, Liu J, Yuan YC. Health information seeking behaviors and source preferences between Chinese and U.S. populations. J Health Commun. 2020;25(6):490–500. doi:10.1080/10810730.2020.1806414
26. Dong F, Wu Y, Wang Q, Huang Y, Wu Q. Factors influencing patient engagement in decision-making for catheter ablation of atrial fibrillation: a cross-sectional survey. Eur J Cardiovasc Nurs. 2025;24(1):150–157. doi:10.1093/eurjcn/zvae141
27. Wang Y, Qiu Y, Ren L, Jiang H, Chen M, Dong C. Social support, family resilience and psychological resilience among maintenance hemodialysis patients: a longitudinal study. BMC Psychiatry. 2024;24(1):76. doi:10.1186/s12888-024-05526-4
28. Wu H, Fan Y, Cheng Y, Zhang JE. Predictors of shared decision-making in patients with recurrent and metastatic nasopharyngeal carcinoma: an observational structural equation modeling approach. Eur J Oncol Nurs. 2025;76:102869. doi:10.1016/j.ejon.2025.102869
29. Rodríguez Del Águila M, González-Ramírez A. Sample size calculation. Allergol Immunopathol. 2014;42(5):485–492. doi:10.1016/j.aller.2013.03.008
30. Nolan MT, Hughes M, Narendra DP, et al. When patients lack capacity: the roles that patients with terminal diagnoses would choose for their physicians and loved ones in making medical decisions. J Pain Symptom Manage. 2005;30(4):342–353. doi:10.1016/j.jpainsymman.2005.04.010
31. Xu X. The patients satisfaction with participation in medical decision-making scale: development, reliability, and validity. 2010.
32. Dong F, Wu Y, Wang Q, Huang Y, Wu Q. Factors influencing patient engagement in decision making for catheter ablation of atrial fibrillation: a cross-sectional survey. Eur J Cardiovasc Nurs. 2024;2024:zvae141.
33. Xiao S. Theoretical basis and research application of social support rating scale. J Clini Psych. 1994;4(2):98.
34. Martin LR, DiMatteo MR, Lepper HS. Facilitation of patient involvement in care: development and validation of a scale. Behav Med. 2001;27(3):111–120. doi:10.1080/08964280109595777
35. Bunn H, O’Connor A. Validation of client decision-making instruments in the context of psychiatry. Can J Nurs Res. 1996;28(3):13–27.
36. Xu X, Mao J, Wang J, Zhao H. Developing strategy and item selection of the patients’ expectation for participation in medical decision making scale. China Mod Med. 2012;19:162–164.
37. Wang Y, Zhang S, Fang W, et al. Factors influencing decision-making preferences among patients with inflammatory bowel disease: a cross-sectional study in China. Patient Prefer Adherence. 2025;19:1047–1057. doi:10.2147/ppa.S517510
38. Abe M, Hashimoto H, Soejima A, et al. Shared decision-making in patients with gynecological cancer and healthcare professionals: a cross-sectional observational study in Japan. J Gynecol Oncol. 2025;36(3):e47. doi:10.3802/jgo.2025.36.e47
39. Scott AW, Bressler NM, Ffolkes S, Wittenborn JS, Jorkasky J. Public attitudes about eye and vision health. JAMA Ophthalmol. 2016;134(10):1111–1118. doi:10.1001/jamaophthalmol.2016.2627
40. Su C, Wang Z, Dong X, Ma X. Experiences of seeking diabetic eye care among patients with diabetes in China: a community-based convergent mixed methods study. Public Health. 2024;234:24–32. doi:10.1016/j.puhe.2024.05.021
41. Muscat DM, Shepherd HL, Nutbeam D, Trevena L, McCaffery KJ. Health literacy and shared decision-making: exploring the relationship to enable meaningful patient engagement in healthcare. J Gen Intern Med. 2021;36(2):521–524. doi:10.1007/s11606-020-05912-0
42. Bravo P, Edwards A, Barr PJ, Scholl I, Elwyn G, McAllister M. Conceptualising patient empowerment: a mixed methods study. BMC Health Serv Res. 2015;15:252. doi:10.1186/s12913-015-0907-z
43. Brabers AE, Rademakers JJ, Groenewegen PP, van Dijk L, de Jong JD. What role does health literacy play in patients’ involvement in medical decision-making? PLoS One. 2017;12(3):e0173316. doi:10.1371/journal.pone.0173316
44. Ousseine YM, Durand MA, Bouhnik AD, Smith A, Mancini J. Multiple health literacy dimensions are associated with physicians’ efforts to achieve shared decision-making. Patient Educ Couns. 2019;102(11):1949–1956. doi:10.1016/j.pec.2019.05.015
45. Mertens L, Kasmi T, Bekkering GE, et al. Shared challenges and opportunities: uncovering common ground in patient participation across different healthcare settings and patient groups. A qualitative meta-summary on patient-reported barriers and facilitators to participation in shared decision-making. Patient Educ Couns. 2025;130:108475. doi:10.1016/j.pec.2024.108475
46. Kraetschmer N, Sharpe N, Urowitz S, Deber RB. How does trust affect patient preferences for participation in decision-making? Health Expect. 2004;7(4):317–326. doi:10.1111/j.1369-7625.2004.00296.x
47. Tefera GM, Ngondwe P, Varol S. Trust matters: a qualitative study on healthcare access and utilization among African immigrants in the United States. J Community Health. 2025. doi:10.1007/s10900-025-01481-7
48. Scherer KA, Büdenbender B, Blum AK, et al. Power asymmetry and embarrassment in shared decision-making: predicting participation preference and decisional conflict. BMC Med Inform Decis Mak. 2025;25(1):120. doi:10.1186/s12911-025-02938-4
49. Street RL, Liu L, Farber NJ, et al. Keystrokes, mouse clicks, and gazing at the computer: how physician interaction with the EHR affects patient participation. J Gen Intern Med. 2018;33(4):423–428. doi:10.1007/s11606-017-4228-2
50. Fenwick E, Rees G, Pesudovs K, et al. Social and emotional impact of diabetic retinopathy: a review. Clin Exp Ophthalmol. 2012;40(1):27–38. doi:10.1111/j.1442-9071.2011.02599.x
51. Xiang JM, Gao LL. Decisional conflict, anxiety, and social support among Chinese pregnant women making further prenatal testing decisions. J Reprod Infant Psychol. 2025;43(1):34–46. doi:10.1080/02646838.2023.2232380
52. Wu M, Wang W, He H, Bao L, Lv P. Mediating effects of health literacy, self-efficacy, and social support on the relationship between disease knowledge and patient participation behavior among chronic Ill patients: a cross-sectional study based on the capability-opportunity-motivation and behavior (COM-B) model. Patient Prefer Adherence. 2025;19:1337–1350. doi:10.2147/ppa.S513375
53. Nguyen TQ, Le TD, Fan SY, Ke LS, Pham TVH, Kao CY. Exploring relational autonomy of vietnamese patients’ experiences in decision-making regarding hematopoietic stem cell transplantation: a qualitative interview study. Psychooncology. 2025;34(6):e70185. doi:10.1002/pon.70185
54. Wang H, Xiu M, Yang F, et al. Perceptions of shared decision-making participation during cardiac rehabilitation in coronary heart disease patients after coronary artery bypass surgery grafting: a qualitative study. BMC Cardiovasc Disord. 2025;25(1):529. doi:10.1186/s12872-025-04996-y
55. Lin ML, Huang CT, Chen CH. Reasons for family involvement in elective surgical decision-making in Taiwan: a qualitative study. J Clin Nurs. 2017;26(13–14):1969–1977. doi:10.1111/jocn.13600
56. Charles C, Whelan T, Gafni A. What do we mean by partnership in making decisions about treatment? BMJ. 1999;319(7212):780–782. doi:10.1136/bmj.319.7212.780
57. Yan S, Wang D, Huang Q, et al. Examining cancer patient preferences during three stages of decision making and family involvement: a multicenter survey study in China. BMC Med Inform Decis Mak. 2025;25(1):9. doi:10.1186/s12911-024-02846-z
58. Salm H, Schuler MK, Hentschel L, et al. Preferences on treatment decision making in sarcoma patients: prevalence and associated factors – results from the PROSa study. Oncol Res Treat. 2025;48(4):174–185. doi:10.1159/000543456
59. Drewniak D, Brandi G, Buehler PK, et al. Key factors in decision making for ECLS: a binational factorial survey. Med Decis Making. 2022;42(3):313–325. doi:10.1177/0272989×211040815
60. Sio TT, Chang K, Jayakrishnan R, et al. Patient age is related to decision-making, treatment selection, and perceived quality of life in breast cancer survivors. World J Surg Oncol. 2014;12:230. doi:10.1186/1477-7819-12-230
61. Ambigapathy R, Chia YC, Ng CJ. Patient involvement in decision-making: a cross-sectional study in a Malaysian primary care clinic. BMJ Open. 2016;6(1):e010063. doi:10.1136/bmjopen-2015-010063

Using AI to transcribe speech is nothing new. Apps such as Otter.ai have been proven to be a true game changer in this regard, allowing audio containing speech to be turned into accurate, readable text in next to no time.
In many cases, however,…
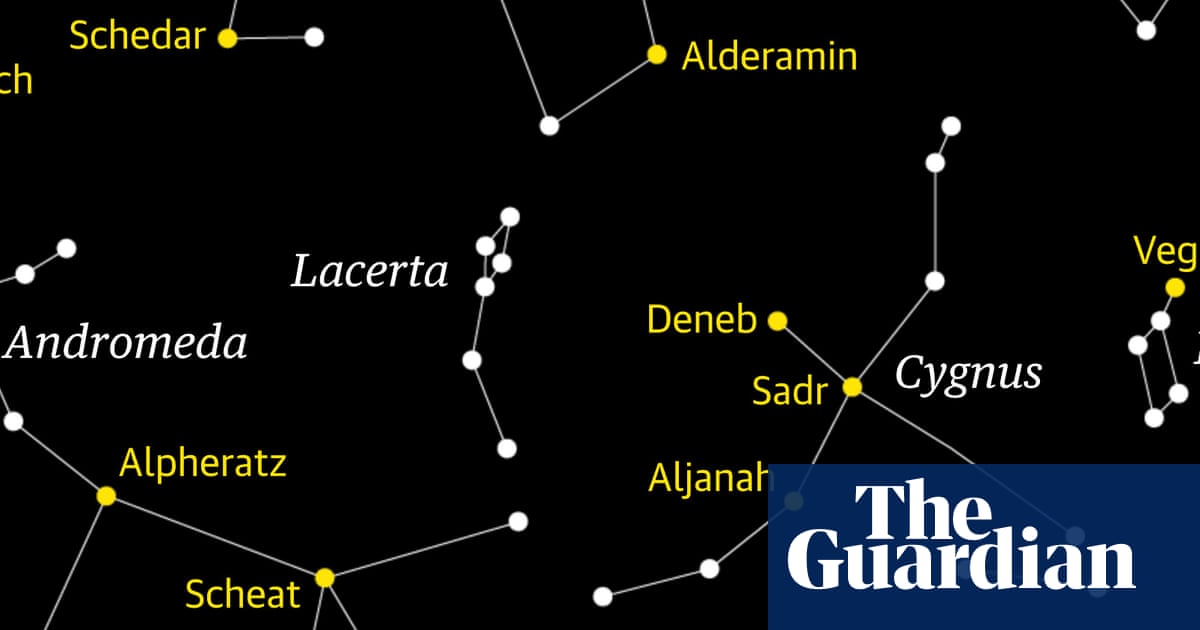
Time to track down a faint gem of the northern skies. Nestled between the bright constellations of Cygnus, the swan, and the mythical mother-daughter pair of Cassiopeia and Andromeda, Lacerta, the lizard, is admittedly a faint…

More than 100,000 cosplayers and comic book fans flocked to Excel London this weekend for MCM Comic-Con. Seeing old friends or making new ones, people young and old bond over shared interests, often feeling a sense of belonging that can be hard…

Shixuan Zhang,1 Panpan Liu,2 Sitong Liu,1 Minghuan He,1 Song Zheng,3 Xiaodong Sun,4 Ruiqun Qi,3,5 Xinghua Gao,3,5 Lili Zhu1
1Department of Dermatology, the People’s Hospital of Liaoning Province, Shenyang, Liaoning Province, 110016, People’s…


Aortic dissection (AD) is a life-threatening cardiovascular disorder with worldwide clinical significance, pathologically defined by an intimal rupture permitting blood entry into the medial layer, consequently creating separate true and false luminal spaces within the aortic wall.1 The disease progression entails substantial architectural changes in the aorta, featuring VSMC loss, ECM breakdown, and leukocyte accumulation.2 Crucially, inflammatory responses play a central mechanistic role in AD development. Invading immune cells (notably lymphocytes and macrophages) enhance protease and adhesion molecule production while releasing reactive oxygen species. These cells additionally trigger VSMC apoptosis, ultimately causing intimal deterioration – the principal pathological process driving AD formation.3 Although current management relying on surgical and endovascular techniques has advanced, the persistent lack of precise molecular targets and effective drug therapies emphasizes the critical necessity for discovering robust biomarkers to enable timely diagnosis and targeted treatment development.
Lactylation, an essential post-translational modification, significantly influences diverse biological pathways. Conventionally, lactate was viewed solely as the end-product of glycolysis.4 However, a paradigm shift occurred in 2019 when Professor Yingming Zhao’s research team identified lactylation as a novel protein modification occurring on lysine residues, thereby revolutionizing research in this field. This enzymatic process, catalyzed by specific enzymes, facilitates the attachment of lactate groups to lysine residues, subsequently modifying protein charge, structure, and function, and ultimately influencing diverse biological processes.5 The implications of lactylation extend across multiple cellular physiological activities. During tumor development, lactylation regulates essential oncogenic processes such as cancer cell metabolism, proliferation, invasion and metastatic spread via functional modulation of proteins.6 Within the cardiovascular domain, lactylation significantly contributes to various inflammatory responses and impacts cardiovascular system functionality.7–9 Moreover, this modification critically governs immune regulation, profoundly altering immune cell functionality and response dynamics.
Given lactylation’s substantial involvement in cardiovascular diseases and inflammation,10–14 coupled with the absence of reported expression and functions of lactylation-associated genes in AD, this study aims to explore potential connections between lactylation-associated genes, AD, and immunological characteristics. This investigation seeks to provide novel directions and insights for early AD diagnosis. Integrative analysis of bulk and single-cell transcriptomic data enabled identification of lactylation-associated genes in AD and uncovered their correlation with immune microenvironment characteristics.This research is anticipated to yield promising biomarkers for AD diagnosis and establish a novel approach for AD detection, thereby contributing to the theoretical foundation and technical support for early and precise diagnosis of aortic dissection.
Transcriptomic profiles from two independent GEO cohorts (GSE52093, n=12; GSE98770, n=11) were integrated for analysis. Corresponding clinical information and normalization files were also acquired from the GEO database. To ensure data consistency, batch effects were addressed through principal component analysis (PCA) and the application of the “ComBat” function from the “SVA” R package. This processing yielded a comprehensive AD cohort comprising 23 samples and 18,249 genes, suitable for subsequent analysis.
Utilizing the integrated dataset, we performed differential expression analysis employing the limma package (version 4.2.1) in RStudio. DEGs were screened using cutoff criteria of |log2FC| > 0.58 (1.5-fold) and adjusted p-value < 0.05, with analytical results presented in volcano and heatmap visualizations.15 Subsequently, systematic functional annotation of significant DEGs was performed via Gene Ontology (GO) analysis and KEGG pathway enrichment.
Based on comprehensive literature review, we identified 323 lactylation-related genes(Supplementary Data Sheet 1). Integration of differential expression results with lactylation-related genes enabled identification of lactylation-associated DEGs (LDEGs) through intersection analysis. Functional annotation of LDEGs was subsequently conducted using R-based clusterProfiler, incorporating GO enrichment and KEGG pathway analyses to delineate their biological roles. The functional enrichment results were visualized with R’s ggplot2 package to facilitate data interpretation.
To elucidate the functional characteristics and metabolic pathways of differentially expressed lactylation-associated genes, we utilized the GeneMANIA database (http://genemania.org), a comprehensive online resource for gene function prediction and interaction network construction. This platform enables rapid generation of gene interaction networks by analyzing input gene lists, thereby elucidating relationships between genes and their interaction partners. GeneMANIA integrates diverse data sources and employs sophisticated network weighting models to predict functions of unknown genes based on their interactions with functionally characterized genes. In our study, we imported the differentially expressed lactylation-related genes into this platform to construct and visualize a protein-protein interaction (PPI) network, facilitating comprehensive analysis of gene interactions and functional associations.
Three machine learning approaches were employed to analyze pre-filtered lactylation-associated DEGs for hub gene identification in AD: (1) LASSO regression via R’s “glmNETs” package; (2) RF and SVM-REF analyses using “randomForest” and “kernlab” packages respectively. Integration of feature genes from all algorithms identified lactylation-related hub genes, followed by ROC curve analysis with AUC calculation (using “pROC”) to assess diagnostic performance for AD.
Conducting a correlation analysis and visualization between the selected hub genes and other differentially expressed genes using the “corrplot” package in R. Subsequently, analyze the differential expression of each hub gene utilizing the Wilcoxon rank-sum test. Finally, perform Gene Set Enrichment Analysis (GSEA) on the Hallmark gene sets (http://software.broadinstitute.org/gsea/msigdb/) for each hub gene using the R package “clusterProfiler” to elucidate the enriched pathways and functions associated with the hub genes.
Existing evidence confirms immune cell involvement in AD pathophysiology. Using CIBERSORT, we profiled infiltration patterns of 22 immune cell subtypes in AD cohorts to examine hub gene-immune cell interactions.16 Immune cell correlations and infiltration variations were displayed via R’s “corrplot” and “ggplot2”, while Spearman analysis (“ggstatsplot”) revealed hub gene-immune cell associations, providing mechanistic insights into AD immunopathology.
External validation using the GSE153434 dataset confirmed hub gene expression profiles, with ROC analysis evaluating their diagnostic potential.
The scRNA-seq dataset (GSE213740: 6 AD cases vs 3 controls) was processed with Seurat in R. Quality control excluded cells with: >15% mitochondrial genes, >3% ribosomal genes, >0.1% erythrocyte genes, or gene counts <200/>7500. Post-QC, PCA-based dimensionality reduction preceded cell clustering (resolution=0.05) and visualization. Cluster-specific DEGs were identified using FindMarkers (log2FC>0.5, p<0.05), with top 5 markers visualized. Cellular lactylation levels were quantified via singscore for AD-normal comparisons.
The inclusion criteria for aortic dissection cases comprised patients who were: (1) diagnosed with aortic dissection through aortic CTA angiography, (2) underwent aortic artificial vessel replacement surgery, and (3) were aged over 18 years. Normal aortic tissues were collected from individuals undergoing coronary artery bypass grafting procedures. This study incorporated a total of nine clinical samples for differential gene expression validation, including six aortic dissection cases and three control cases (with aortic wall tissue obtained from the perforation site of the ascending aorta during coronary artery bypass grafting). The study protocol was conducted in strict compliance with the ethical principles outlined in the Helsinki Declaration. All experimental procedures and study protocols were reviewed and approved by the Ethics Committee of the Affiliated Hospital of Nantong University (Ethical approval number:2024-K247-01), and written informed consent was obtained from all participants prior to their inclusion in the study.
Total RNA extraction from aortic tissues was conducted with TRIzol reagent (ACCURATE BIOTECHNOLOGY). cDNA synthesis utilized HiScript II Q RT SuperMix (+gDNA wiper, Vazyme R223-01). qPCR amplification was performed with ChamQ SYBR Master Mix (Vazyme Q311-02), normalized to β-Actin expression (primers in Table 1). The 2–ΔΔCT method calculated relative expression, with p<0.05 considered statistically significant.
|
Table 1 Primer Sequences of Hub Genes
|
To advance the identification of prospective therapeutic agents for aortic dissection, this research leveraged the Drug-Gene Interaction Database (DGIdb) to pinpoint targeted drugs corresponding to critical biological targets. The drugs that received the highest scores from this prediction were subsequently subjected to validation through molecular docking experiments with those key targets. To facilitate this process, the two-dimensional structures of small molecule ligands were sourced from the PubChem database (http://pubchem.ncbi.nlm.nih.gov/). These 2D structures were then converted into three-dimensional representations using ChemOffice software, and the resulting 3D structures were saved in mol2 file format for further analysis. For the molecular docking studies, high-resolution crystal structures of the relevant protein targets were obtained from the RCSB Protein Data Bank (RCSB PDB) (http://www.rcsb.org/). These structures were utilized as the docking receptors in our simulations. To prepare the proteins for the docking process, PyMOL software was employed to eliminate water molecules and phosphate groups, resulting in cleaned protein structures that were saved as PDB files. The molecular docking itself was conducted using AutoDock Vina 1.5.6 software, which facilitated the investigation of interactions between the proteins and the ligands. Throughout this process, the structures of both the proteins and the small molecule ligands underwent several modifications; hydrogen atoms were added to the proteins, water molecules were removed, and hydrogen atoms, along with specific torsional degrees of freedom for the small molecule ligands, were carefully managed. Following these preparations, the docking box coordinates were established.Finally, by analyzing and comparing the docking scores, the most favorable conformation from the molecular simulations was ultimately identified. Visualization and analysis of the interaction patterns between the candidate compounds and critical amino acids were conducted using PyMOL and Discovery Studio 2019 software, elucidating the 2D and 3D interaction diagrams that are crucial for understanding the binding characteristics of the predicted therapeutic agents.
After integrating and removing batch effects from two AD-related datasets (GSE52093 and GSE98770), we achieved expression profiles that included 13 patients diagnosed with AD and 10 healthy individuals (Figure 1A–D). The analysis for differential expression found 423 genes that were upregulated and 470 that were downregulated in patients with AD (Figure 2A). The 50 genes with the most significant differential expression were depicted using hierarchical clustering in a heatmap (Figure 2B). Following this, GO enrichment analysis of the differentially expressed genes (Figure 3A) indicated notable enrichment in biological processes linked to “mitotic cell cycle phase transition”, cellular components related to “collagen-containing extracellular matrix”, and molecular functions primarily associated with “actin binding.” Furthermore, an analysis of pathways using the KEGG revealed significant connections between AD and both the “cell cycle” as well as the “p53 signaling pathway” (Figure 3B).
 |
Figure 1 Analysis of the merged and corrected two GEO datasets. (A and B) Expression profiles before and after raw data correction. (C and D) Differential gene expression profiles before and after data normalization.
|
 |
Figure 2 Differential expression analysis of the AD patient dataset. (A) Volcano plot of differentially expressed genes. (B) Heatmap of the top 50 differentially expressed genes.
|
 |
Figure 3 Functional enrichment analysis of differentially expressed genes. (A) GO enrichment analysis. (B) KEGG enrichment analysis.
|
Following the acquisition of expression profiles, we examined 332 lactylation-associated genes and their differential expression patterns. The analysis revealed that, in contrast to three downregulated genes, ten lactylation-related genes were significantly upregulated in Alzheimer’s disease (AD) patients (Figure 4A and B). In order to substantiate these findings further, we conducted a functional analysis on 13 genes associated with lactylation that were expressed differentially. The GO analysis revealed notable enrichment in biological processes concerning “protein localization within organelles”, molecular functions related to “binding specific to protein domains”, and cellular components tied to “histone deacetylase binding” (Figure 4C–E). Furthermore, the KEGG pathway analysis uncovered significant changes in the pathways of “vascular smooth muscle contraction” and “cellular senescence” (Figure 4F).
 |
Figure 4 Functional Analysis of Lactylation-Related Genes. (A and B) Differentially expressed genes related to lactylation. (C–E). GO enrichment analysis of lactylation-related genes. (F) KEGG enrichment analysis of lactylation-related genes.
|
To systematically identify hub genes associated with lactylation, we employed a multi-method approach. First, LASSO regression analysis was performed on the 13 differentially expressed genes, yielding 5 feature genes (Figure 5A and B). Subsequently, random forest ranking was applied to prioritize lactylation-related genes, identifying the top 10 candidates (Figure 5C). Further refinement using the SVM-REF algorithm revealed 4 feature genes (Figure 5D). Through integration of results from these three complementary methods, we identified three hub genes for lactylation: CALM1, PTBP1, and PARP1 (Figure 5E). The diagnostic potential of these hub genes was evaluated using ROC analysis, demonstrating their robust ability to distinguish AD patients from healthy controls (Figure 5F). Finally, we constructed a protein-protein interaction network for these three differentially expressed lactylation-related hub genes using the GeneMANIA database (Supplementary Figure 1).
 |
Figure 5 Identification of Lactylation Hub Genes. (A–B) LASSO regression identified 5 feature genes. (C) Random Forest ranked the importance of 10 feature genes. (D) SVM support vector machine algorithm selected 4 feature genes. (E) The intersection of feature genes obtained from the three methods yielded 3 hub genes. (F) ROC curves of the 3 genes for predicting disease occurrence.
|
After identifying the hub genes, we performed comprehensive correlation analyses between these three hub genes and all other genes, visualizing the top three genes showing significant positive and negative correlations. Our findings revealed that RILPL1, PLS3, and GABARAPL2 exhibited significant positive correlations with CALM1, while TRIP12, SMARCC1, and CYB561D2 showed significant negative correlations with CALM1 (Supplementary Figure 2). Similarly, FES, TFDP1, and ELF3 demonstrated significant positive correlations with PARP1, whereas DNAJC24, DIXDC1, and AFTPH displayed significant negative correlations with PARP1 (Supplementary Figure 3). Furthermore, CANT1, ILF3, and XPO6 were significantly positively correlated with PTBP1, while CRBN, CD2AP, and TMEM106B showed significant negative correlations with PTBP1 (Supplementary Figure 4).
Subsequently, we investigated the roles of CALM1, PARP1, and PTBP1 in AD by comparing their expression profiles between normal and AD groups. The findings indicated that CALM1 levels were significantly elevated in the normal group in comparison to the AD group. Conversely, expression levels of PARP1 and PTBP1 were significantly increased in the AD group when compared to the normal group (Figure 6A). Additionally, we performed Gene Set Enrichment Analysis (GSEA) on these central genes (Figure 6B). This analysis uncovered a positive correlation between CALM1 levels and the myogenesis pathway, alongside a negative correlation with the glycolysis and oxidative phosphorylation pathways. Meanwhile, PARP1 expression demonstrated positive links to the NF-κB signaling pathway and the inflammatory response pathway, while showing a negative relationship with the myogenesis pathway. In a similar manner, PTBP1 expression was positively associated with both the NF-κB signaling and inflammatory response pathways, but negatively associated with the myogenesis pathway.
 |
Figure 6 Characterization of Hub Gene Expression and GSEA Enrichment Analysis. (A) Expression of hub genes, with red indicating the normal group and blue representing the AD group. (B) GSEA enrichment results of hub genes.
|
We conducted a comprehensive analysis of immune cell infiltration levels in patients with aortic dissection (AD) and healthy controls. The results revealed that healthy individuals exhibited significantly higher infiltration levels of γδT cells and resting mast cells compared to the AD group, while showing significantly lower levels of resting natural killer cell infiltration (Figure 7A and C). To further investigate the interrelationships among immune cells, we performed correlation analysis to elucidate potential interactions and their implications for immune-inflammatory mechanisms in AD (Figure 7B).
 |
Figure 7 Immunoinfiltration Analysis. (A) The relative proportions of 22 immune cell subsets in all samples from the AD dataset. (B) The correlations of the 22 immune cell subsets. (C) Differences in the levels of 22 immune cell types between the normal group and the AD group.
|
Our analysis demonstrated a positive correlation between γδT cells and both M1 macrophages and resting mast cells, suggesting potential synergistic interactions and functional interdependence among these cell populations in the immune response to aortic dissection. Conversely, we observed negative correlations between resting natural killer cells and both resting mast cells and γδT cells, potentially reflecting disease-induced imbalances in the immune system’s inflammatory response, immune regulation, and immune surveillance capabilities. These findings highlight the crucial role of immune cell interactions in the pathogenesis and progression of aortic dissection.
Furthermore, we identified significant correlations between the three hub genes and specific immune cell populations (Figure 8). CALM1 expression showed a significant negative correlation with plasma cell infiltration, while demonstrating positive correlations with CD8-positive T cells, resting mast cells, and γδT cells. PARP1 expression exhibited a negative correlation with γδT cell infiltration and positive correlations with CD4-positive naive T cells and resting NK cells. PTBP1 expression was negatively correlated with γδT cell infiltration and positively correlated with resting NK cells, activated dendritic cells, monocytes, and naive B cells. These findings suggest complex interactions between lactylation-related genes and immune cell populations in the context of aortic dissection.
 |
Figure 8 Correlation between Hub Genes and Immune Cells.
|
To assess the reliability of hub genes related to lactylation that are differentially expressed in AD, we analyzed the expression profiles of three lactylation-associated genes—CALM1, PTBP1, and PARP1—using the independent dataset GSE153434. Our comparative analysis indicated that the expression of CALM1 was significantly reduced in the AD group when compared to the normal control group. In contrast, we found that PTBP1 expression was significantly increased in the AD group relative to the normal controls (Figure 9A and B). Interestingly, PARP1 expression did not demonstrate any notable differences between the two groups (Figure 9C). The receiver operating characteristic (ROC) curve analysis revealed that both CALM1 and PTBP1 had strong diagnostic capabilities, with area under the curve (AUC) values of 0.93 and 0.83, respectively. On the other hand, PARP1 exhibited minimal diagnostic value, yielding an AUC of 0.50 (Figure 9D).
 |
Figure 9 External Dataset Validation of Hub Genes. (A–C) Expression characteristics of hub genes in the external dataset, with red representing the normal group and blue representing the AD group. (D) ROC curves of hub genes for predicting disease occurrence in the external dataset.
|
The single-cell dataset GSE213740, comprising six aortic dissection samples and three normal controls, was obtained from the GEO database. Initial quality control measures were implemented, with Figure 10A and B illustrating the distribution of gene counts (nFeature), sequencing depth (nCount), and mitochondrial gene percentage (percent.mt). For subsequent analysis, the top 2000 highly variable genes were selected, with the top 10 most variable genes displayed in Figure 10C. Based on the analysis presented in Figure 11A, we determined the optimal number of principal components (PCs) to be 9. Cluster analysis results suggested an appropriate resolution of 0.1 (Figure 11B), with marker genes for each cell population visualized through heatmap analysis (Figure 12A). Utilizing the UMAP algorithm, we classified the single-cell sequencing samples into 11 distinct clusters (Figure 12B), which were subsequently identified as 11 immune cell populations (Figure 12C). These populations included endothelial cells, smooth muscle cells, mesenchymal cells, macrophages, T cells, B cells, monocytes, mast cells, plasma cells, fibroblasts, M1 macrophages, and M2 macrophages. Differential expression analysis across all 11 cell populations identified the top five highly and lowly expressed genes in each population (Figure 13A).
 |
Figure 10 Data Quality Control. (A and B) Changes in single-cell dataset quality control before and after. (C) Top 10 highly variable gene markers.
|
 |
Figure 11 Selection of Principal Component Numbers and Resolution. (A) Heatmap is used to display the expression levels of marker genes for the top 20 PCs. (B) Clustering tree is used to select the appropriate resolution.
|
 |
Figure 12 Cell Population Clustering. (A) Heatmap of marker genes for each cell population. (B) UMAP algorithm divides cells into 11 subgroups. (C) 11 cell subgroups are annotated as 11 immune cell types.
|
 |
Figure 13 Differential Expression Analysis. (A) Differentially expressed genes (top 5) in each cell cluster. (B) Expression profiles of hub genes across different cell clusters.
|
Further investigation of lactylation hub gene expression across these 11 immune cell types revealed distinct expression patterns (Figure 13B). CALM1 exhibited high expression in smooth muscle cells, B cells, and fibroblasts, while showing low expression in endothelial cells, mesenchymal cells, mast cells, and M2 macrophages. PTBP1 demonstrated elevated expression in endothelial cells, T cells, B cells, mast cells, fibroblasts, and M1 macrophages, but was minimally expressed in smooth muscle cells, mesenchymal cells, monocytes, and M2 macrophages. Similarly, PARP1 showed high expression levels in T cells, B cells, fibroblasts, and M1 macrophages, with reduced expression observed in mesenchymal cells, monocytes, and mast cells.
To further investigate the immune-related interactions between lactylation and AD, we performed single-cell scoring analysis of the lactylation-related gene set. Our analysis revealed elevated lactylation levels in fibroblasts, smooth muscle cells, monocytes, and T cells, while other immune cell types showed no significant differences in lactylation levels (Supplementary Figure 5A). Subsequent comparative analysis of lactylation gene set scores between AD and normal samples demonstrated significantly higher lactylation scores in the AD group. This finding suggests that aortic tissues from AD patients exhibit elevated lactylation levels compared to normal controls. Furthermore, we observed significant differences in lactylation levels of immune cells between the two groups (Supplementary Figure 5B).
RT-qPCR was utilized to assess the expression profiles of CALM1, PTBP1, and PARP1 in clinical samples, with comparisons made between patients with aortic dissection (AD) (n = 6) and normal controls (n = 3). The results indicated a notable decrease in CALM1 expression within the AD cohort relative to the normal controls. Conversely, the expression levels of PTBP1 and PARP1 did not exhibit any statistically significant variations between the AD and control groups (Figure 14).
 |
Figure 14 RT-qPCR detection of hub gene mRNA expression levels. ns, no significant difference, *p < 0.05.
|
Utilizing the DGIdb database, we forecasted potential drugs aimed at the key biomarker CALM1 and ultimately pinpointed the top five highest-scoring candidate drugs (Figure 15A). Among these candidates, PRENYLAMINE, which showed the highest targeting score, was chosen for molecular docking validation against CALM1. Generally, a binding energy that falls below −5.0 kcal/mol is indicative of favorable binding activity between the ligand and its target protein, where lower binding energy values signify greater binding affinity, increased stability, and more advantageous conformational interactions. Our findings revealed that PRENYLAMINE reached a binding energy of −6.6 kcal/mol when interacting with CALM1 (Figure 15B), indicating its potential as a therapeutic option for aortic dissection.
 |
Figure 15 Drug Prediction and Molecular Docking Validation.(A) Potential targeted drugs for CALM1.(B) Molecular docking results of PRENYLAMINE with CALM1.
|
The current understanding of aortic dissection (AD) pathogenesis recognizes inflammation, apoptosis, endothelial cell dysfunction, phenotypic transformation of smooth muscle cells, and extracellular matrix degradation as critical pathological components, with emerging evidence implicating epigenetic modifications in these processes.17–21 While metabolic reprogramming and its epigenetic consequences are increasingly recognized in cardiovascular pathologies, the specific role of lactate accumulation, and its associated lactylation modifications in AD development remains unexplored. Our study bridges this knowledge gap by identifying three lactylation-associated hub genes (CALM1, PARP1, and PTBP1) through integrated RNA sequencing analysis and investigated their relationship with immune cell infiltration patterns.
Firstly, through comprehensive transcriptomic analysis, we identified CALM1 as a potential lactylation-related biomarkers distinguishing AD patients from healthy individuals. As the most evolutionarily conserved calmodulin isoform,22,23 CALM1 regulates fundamental cellular process including motility, differentiation, and proliferation,24 with established roles in oncogenesis,25 neurodegeneration,26 and fibrotic disorders.27 Mechanistically, CALM1 overexpression activates PI3K-Akt pathway, subsequently suppressing hepatic gluconeogenesis – a pathway critically involved in apoptosis regulation, oxidative stress responses, and inflammatory signaling.28
Our network analysis extends these finding by revealing novel association between CALM1 expression and mTORC1 signaling pathway, glycolysis – oxidative phosphorylation coupling, and the infiltration patterns of nearly all immune cell types. These associations suggest CALM1 as a potential orchestrator of the pro-inflammatory microenvironment characteristic of AD. However, further experimental investigations are necessary to fully elucidate the specific functions and molecular mechanisms of CALM1 in the pathogenesis of AD.
PARP1, also known as Poly(ADP-ribose) polymerase 1, is an enzyme that is highly conserved and dependent on NAD⁺. It is crucial for the regulation of various intracellular physiological processes, such as the repair of DNA damage, the regulation of gene transcription, chromatin remodeling, and the modulation of apoptosis.29–31 The relationship between PARP1 and lactate/lactylation is both intricate and significant. Lactate exerts substantial influence on PARP1, not only by directly inhibiting its activity but also by indirectly modulating its function through alterations in cellular energy metabolism and redox balance.32 In chronic inflammation, excessive PARP1 activation establishes a pathogenic feedback loop by amplifying NF-κB-mediated TNFα/IL-6 production.33,34 Our findings demonstrate elevated PARP1 expression levels in aortic tissues of AD patients compared to normal controls. This correlations between PARP1 elevation and CD4T cell/mast cell infiltration align with the previous paradigm, suggesting PARP1-mediated immune dysregulation contributes to AD progression.
The RNA-binding protein PTBP1 emerged as a third lactylation-associated factor elevated in AD tissues. As one member of heterogeneous nuclear ribonucleoprotein (hnRNP) subfamily, PTBP1is encoded on human chromosome 19p13.3.35 In the metabolism of cancer cells, PTBP1 enhances the production of the M2 isoform of pyruvate kinase (PKM2) and concurrently inhibits the expression of PKM1, resulting in a metabolic transition from oxidative phosphorylation to glycolysis, which enhances glycolytic flux and lactate accumulation, significantly influencing tumor initiation and progression.36,37 Meanwhile, recent studies identifies lactylation-modified PTBP1 as a glycolysis amplifier in glioma stem cells via PFKFB4 mRNA stabilization.38
Our single-cell analysis reveals broad PTBP1 upregulation across AD aortic cell types (endothelial cells, T cells, B cells, mast cells, fibroblasts, and M1 macrophages), suggesting it may similarly drive glycolytic flux and lactate accumulation in aortic tissues. The resultant lactylation surge could destabilize immune homeostasis through post-translational modification of regulatory proteins – a hypothesis requiring experimental verification. These findings demonstrate that PTBP1 likely functions as a promoting factor in the pathogenesis and progression of aortic dissection.
Recent advancements in epigenetic research have demonstrated that lactate-induced histone lactylation plays a significant role in modulating cellular physiological functions and immune cell modifications.39 To investigate this phenomenon in AD, we conducted a comprehensive analysis of cellular lactylation levels using single-cell sequencing data, comparing AD patients with healthy individuals. The results revealed significant lactylation elevation in AD lesions, particularly within fibroblasts, smooth muscle cells, and myeloid populations. While B/T lymphocytes exhibited relatively lower lactylation levels, their functional sensitivity to lactylation-mediated epigenetic changes warrants further investigation. The spatial correlation between lactate accumulation (from enhanced glycolysis) and lactylation patterns suggests a self-reinforcing metabolic-epigenetic circuit in AD pathogenesis.
Finally, we performed experimental validation of the three hub genes using clinical samples, which demonstrated statistically significant differences in CALM1 expression between AD and control groups, whereas PARP1 and PTBP1 showed no significant differential expression. This divergence highlights the complex translation from bioinformatic prediction to biomarker validation, potentially reflecting post-transcriptional regulation or tissue-specific expression patterns. These results indicate that CALM1, rather than PARP1 or PTBP1, may serve as a promising biomarker for AD diagnosis.
While our multi-modal analysis implicates lactylation in AD-associated immune dysregulation, several limitations must be acknowledged: 1) The observational nature of human tissue studies precludes causal inference; 2) Lactylation’s cell-type-specific effects require functional validation in experimental models; 3) The diagnostic utility of lactylation markers needs prospective clinical evaluation.
Through a multidisciplinary investigation combining bioinformatic analyses and experimental validation, we conducted a systematic exploration of lactylation-mediated regulatory mechanisms in AD pathogenesis. Our findings demonstrate that CALM1 emerges as a novel and promising diagnostic biomarker, showing robust discriminative capacity between AD patients and healthy controls through comprehensive validation across multiple analytical platforms. Complementing these discoveries, high-resolution single-cell RNA sequencing revealed cell type-specific lactylation patterns within the AD aortic microenvironment, delineating previously unrecognized epigenetic regulatory networks across diverse cellular subpopulations. These results substantially advance our understanding of metabolic-epigenetic crosstalk in vascular remodeling disorders, providing mechanistic insights that bridge lactylation dynamics with AD progression. The identification of CALM1 as a lactylation-associated diagnostic indicator, coupled with our comprehensive cellular atlas of lactylation modifications, establishes a critical foundation for future investigations into AD’s molecular pathology. This work not only elucidates new pathophysiological dimensions of aortic dissection but also presents tangible translational applications, potentially enabling the development of lactylation-targeted diagnostic panels and therapeutic interventions for this life-threatening cardiovascular condition.
The datasets supporting the conclusions of this article are available in the GEO database, https://www.ncbi.nlm.nih.gov/geo/.
All experimental procedures and study protocols were reviewed and approved by the Ethics Committee of the Affiliated Hospital of Nantong University.All studies were conducted in accordance.
This work was supported by grants from Jiangsu Provincial Research Hospital (YJXYY202204-YSB58).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Zhiming Yu, Yue Pan and Xiaoyu Qian contribute equally to this article as co-first author.
1. Vilacosta I, San Román JA, Di Bartolomeo R, et al. Acute aortic syndrome revisited: jacc state-of-the-art review. J Am Coll Cardiol. 2021;78(21):2106–2125. doi:10.1016/j.jacc.2021.09.022
2. Chen Y, Zhang T, Yao F, et al. Dysregulation of interaction between LOXhigh fibroblast and smooth muscle cells contributes to the pathogenesis of aortic dissection. Theranostics. 2022;12(2):910–928. doi:10.7150/thno.66059
3. Luo F, Zhou XL, Li JJ, et al. Inflammatory response is associated with aortic dissection. Ageing Res Rev. 2009;8(1):31–35. doi:10.1016/j.arr.2008.08.001
4. Chen AN, Luo Y, Yang YH, et al. Lactylation, a novel metabolic reprogramming code: current status and prospects. Front Immunol. 2021;12:688910. doi:10.3389/fimmu.2021.688910
5. Zhang D, Tang Z, Huang H, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575–580. doi:10.1038/s41586-019-1678-1
6. Malsi K, Xie S, Cai Y, et al. The role of lactylation in tumor growth and cancer progression. Front Oncol. 2025;15. doi:10.3389/fonc.2025.1516785
7. She H, Hu Y, Zhao G, et al. Dexmedetomidine ameliorates myocardial ischemia-reperfusion injury by inhibiting MDH2 lactylation via regulating metabolic reprogramming. Adv Sci. 2024;11(48):e2409499. doi:10.1002/advs.202409499
8. Wang J, Yang P, Yu T, et al. Lactylation of PKM2 suppresses inflammatory metabolic adaptation in pro-inflammatory macrophages. Int J Biol Sci. 2022;18(16):6210–6225. doi:10.7150/ijbs.75434
9. Wu D, Spencer CB, Ortoga L, et al. Histone lactylation-regulated METTL3 promotes ferroptosis via m6A-modification on ACSL4 in sepsis-associated lung injury. Redox Biol. 2024;74:103194. doi:10.1016/j.redox.2024.103194
10. Zhang N, Zhang Y, Xu J, et al. α-myosin heavy chain lactylation maintains sarcomeric structure and function and alleviates the development of heart failure. Cell Res. 2023;33(9):679–698. doi:10.1038/s41422-023-00844-w
11. Li X, Chen M, Chen X, et al. TRAP1 drives smooth muscle cell senescence and promotes atherosclerosis via HDAC3-primed histone H4 lysine 12 lactylation. Eur Heart J. 2024;45(39):4219–4235. doi:10.1093/eurheartj/ehae379
12. Xu X, Zhang DD, Kong P, et al. Sox10 escalates vascular inflammation by mediating vascular smooth muscle cell transdifferentiation and pyroptosis in neointimal hyperplasia. Cell Rep. 2023;42(8):112869. doi:10.1016/j.celrep.2023.112869
13. An S, Yao Y, Hu H, et al. PDHA1 hyperacetylation-mediated lactate overproduction promotes sepsis-induced acute kidney injury via Fis1 lactylation. Cell Death Dis. 2023;14(7):457. doi:10.1038/s41419-023-05952-4
14. Lin J, Ren J. Lactate-induced lactylation and cardiometabolic diseases: from epigenetic regulation to therapeutics. Biochim Biophys Acta Mol Basis Dis. 2024;1870(6):167247. doi:10.1016/j.bbadis.2024.167247
15. Phipson B, Wu D, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi:10.1093/nar/gkv007
16. Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi:10.1038/nmeth.3337
17. Cui H, Chen Y, Li K, et al. Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection. Eur Heart J. 2021;42(42):4373–4385. doi:10.1093/eurheartj/ehab605
18. Luo S, Kong C, Zhao S, et al. Endothelial HDAC1-ZEB2-NuRD complex drives aortic aneurysm and dissection through regulation of protein S-Sulfhydration. Circulation. 2023;147(18):1382–1403. doi:10.1161/CIRCULATIONAHA.122.062743
19. Yin Z, Zhang J, Zhao M, et al. EDIL3/Del-1 prevents aortic dissection through enhancing internalization and degradation of apoptotic vascular smooth muscle cells. Autophagy. 2024;20(11):2405–2425. doi:10.1080/15548627.2024.2367191
20. Chakraborty A, Li Y, Zhang C, et al. Epigenetic induction of smooth muscle cell phenotypic alterations in aortic aneurysms and dissections. Circulation. 2023;148(12):959–977. doi:10.1161/CIRCULATIONAHA.123.063332
21. Rombouts KB, van Merrienboer TAR, Ket JCF, et al. The role of vascular smooth muscle cells in the development of aortic aneurysms and dissections. Eur J Clin Invest. 2022;52(4):e13697. doi:10.1111/eci.13697
22. Ikura M, Ames JB. Genetic polymorphism and protein conformational plasticity in the calmodulin superfamily: two ways to promote multifunctionality. Proceedings National Academy Sci United States Am. 2006;103(5):1159–1164. doi:10.1073/pnas.0508640103
23. Zhang M, Abrams C, Wang L, et al. Structural basis for calmodulin as a dynamic calcium sensor. structure. Structure. 2012;20(5):911–923. doi:10.1016/j.str.2012.03.019
24. Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 2000;10(8):322–328. doi:10.1016/S0962-8924(00)01800-6
25. Yao M, Fu L, Liu X, et al. In-Silico multi-omics analysis of the functional significance of calmodulin 1 in multiple cancers. Front Genet. 2022;12:793508. doi:10.3389/fgene.2021.793508
26. Esteras N, Alquézar C, de la Encarnación A, et al. Calmodulin levels in blood cells as a potential biomarker of Alzheimer’s disease. Alzheimers Res Ther. 2013;5(6):55. doi:10.1186/alzrt219
27. Ji D, Chen GF, Wang JC, et al. Identification of TAF1, HNF4A, and CALM2 as potential therapeutic target genes for liver fibrosis. J Cell Physiol. 2019;234(6):9045–9051. doi:10.1002/jcp.27579
28. Ko HL, Ren EC. Functional aspects of PARP1 in DNA repair and transcription. Biomolecules. 2012;2(4):524–548. doi:10.3390/biom2040524
29. Hussain M, Khadka P, Pekhale K, et al. RECQL4 requires PARP1 for recruitment to DNA damage, and PARG dePARylation facilitates its associated role in end joining. Exp Mol Med. 2025;57(1):264–280. doi:10.1038/s12276-024-01383-z
30. Swindall AF, Stanley JA, Yang ES. PARP-1: friend or Foe of DNA damage and repair in tumorigenesis? Cancers. 2013;5(3):943–958. doi:10.3390/cancers5030943
31. Conceição CJF, Moe E, Ribeiro PA, et al. PARP1: a comprehensive review of its mechanisms, therapeutic implications and emerging cancer treatments. Biochim Biophys Acta Rev Cancer. 2025;11(2):189282. doi:10.1016/j.bbcan.2025.189282
32. Wang L, Liang C, Li F, et al. PARP1 in carcinomas and PARP1 inhibitors as antineoplastic drugs. Int J Mol Sci. 2017;18(10):2111. doi:10.3390/ijms18102111
33. Demény MA, Virág L. The PARP enzyme family and the hallmarks of cancer part 2: hallmarks related to cancer host interactions. Cancers. 2021;13(9):2057. doi:10.3390/cancers13092057
34. Won SJ, Jang BG, Yoo BH, et al. Prevention of acute/severe hypoglycemia-induced neuron death by lactate administration. J Cereb Blood Flow Metab. 2012;32(6):1086–1096. doi:10.1038/jcbfm.2012.30
35. Pina JM, Hernandez LA, Keppetipola NM. Polypyrimidine tract binding proteins PTBP1 and PTBP2 interact with distinct proteins under splicing conditions. PLoS One. 2022;17(2):e0263287. doi:10.1371/journal.pone.0263287
36. Shinohara H, Kumazaki M, Minami Y, et al. Perturbation of energy metabolism by fatty-acid derivative AIC-47 and imatinib in BCR-ABL-harboring leukemic cells. Cancer Lett. 2016;371(1):1–11. doi:10.1016/j.canlet.2015.11.020
37. He X, Arslan AD, Ho TT, et al. Involvement of polypyrimidine tract-binding protein (PTBP1) in maintaining breast cancer cell growth and malignant properties. Oncogenesis. 2014;3(1):e84. doi:10.1038/oncsis.2013.47
38. Zhou Z, Yin X, Sun H, et al. PTBP1 lactylation promotes glioma stem cell maintenance through PFKFB4-Driven glycolysis. Cancer Res. 2025;85(4):739–757. doi:10.1158/0008-5472.CAN-24-1412
39. Gu J, Zhou J, Chen Q, et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-β signaling in regulatory T cells. Cell Rep. Cell Reports. 2022;39(12):110986. doi:10.1016/j.celrep.2022.110986

Type: Interstellar comet (third confirmed visitor from beyond our solar system)
Origin: Likely ejected from a distant star system millions of years ago
Path: “Hyperbolic” trajectory — one-time pass through…